CE,ISO,FDA(510K)
Home > Company Introduction
Company Overview
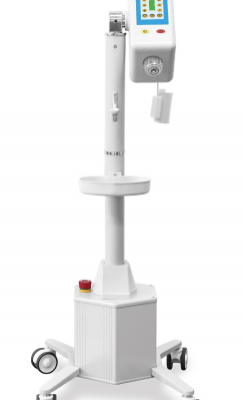
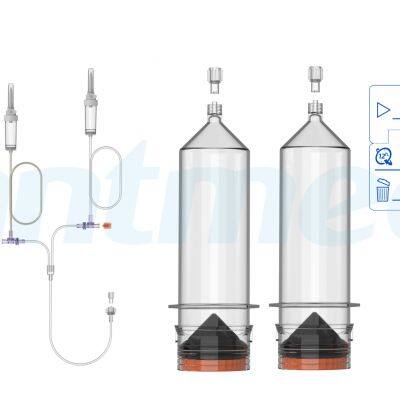
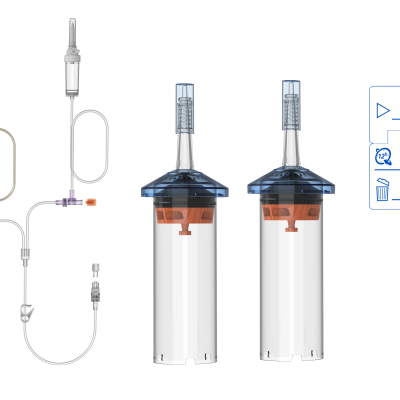
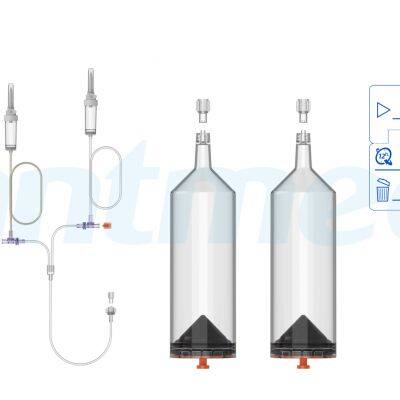
ANTMED is the contrast imaging industry leader in China and the only domestic one-stop solution provider of CT, MR and DSA contrast media power injectors and disposables. Two of our best-selling products are High Pressure Syringes and Disposable Pressure Transducers. Domestically, ANTMED has the largest market share for High Pressure Syringes and is the No.1 manufacturer of Disposable Pressure Transducers. In the global market, ANTMED has customers in 87 countries and regions across the Americas, Europe, Oceania, Africa, Middle-East and South-east Asia.Please feel free to contact us at sindy@antmed.com or whatsapp/wechat 0086 13192081175 if you are interested to be our partner.sindy@antmed.com

-
ManufacturerBusiness Type
-
2000Year Established
-
10,000-30,000 square metersFactory Size
-
Above US$100 MillionAnnual Export Value

Company Detail
Transparency is the foundation of our partnership. Below is a comprehensive overview of our operational metrics, from business scale to global market reach, giving you a clear picture of our capabilities.
-
301 - 500 People Total Employees
-
10-20 People Quality Inspectors
-
Others Product Certifications
-
Above US$100 Million Annual Export Value
-
North America, South America, Eastern Europe, Southeast Asia, Africa, Oceania, Mid East, Eastern Asia, Western Europe, Central America, Northern Europe, Southern Europe, South Asia, Domestic Market Main Markets
-
shenzhen, guangzhou Port of Shipment
-
Medical Diagnosis Equipment Main Industry
-
 Quality Control
Quality Control -
 Our ServicesOur Vision To be a globally respected company in the medical device industry. Our Mission Focus on cutting - edge product innovation in healthcare. Values To be an ethical & responsible business. that will value our employees and grow with our partners. Quality Policy Establish a customer - centric QMS to provide high-quality products and service.
Our ServicesOur Vision To be a globally respected company in the medical device industry. Our Mission Focus on cutting - edge product innovation in healthcare. Values To be an ethical & responsible business. that will value our employees and grow with our partners. Quality Policy Establish a customer - centric QMS to provide high-quality products and service. -
 Company HistoryMarch 2004 - Shenzhen Ant Hi-Tech the predecessor of the Company, was incorporated. July 2005 - The Company obtained the registration certificate for launching its CMPI high pressure syringes and pressure connecting tubes in China. April 2006 - The Company obtained the registration certificate for launching its CMPI in China. May 2008 - The Company obtained FDA510(k) Certificate issued by the FDA for its CMPI angiographic syringes. February 2009 - The Company obtained the registration certificate for launching its disposable pressure transducers in China. August 2009 - The Company obtained FDA510(k) Certificate issued by the FDA for its disposable pressure transducers. July 2011 - The Company obtained FDA510(k) Certificate issued by the FDA for its inflation devices and inflation device compact pack. 2011 - Annual revenue of the Company reached RMB100.0 million. May 2012 - The Company obtained the registration permission for its CMPI high pressure syringes to launch in Canada. September 2012 - The Company obtained a new National High-tech Enterprise Certificate. February 2014 - The Company obtained FDA510(k) Certificate issued by the FDA for its pressure connecting tubes. August 2014 - The Company obtained the registration certificate for launching its dental implant systems in China. . June 2015 - The Company obtained a new National High-tech Enterprise Certificate. July 2015 - The Company completed the construction of its research, development and production facility located in Pingshan New District, Shenzhen. 2017 - Annual revenue of the Company reached RMB300.0 million. February 2018 - Shenzhen Ant Hi-Tech Industrial Co., Ltd. is now a joint stock limited company under the new name Shenzhen Antmed Co., Ltd.
Company HistoryMarch 2004 - Shenzhen Ant Hi-Tech the predecessor of the Company, was incorporated. July 2005 - The Company obtained the registration certificate for launching its CMPI high pressure syringes and pressure connecting tubes in China. April 2006 - The Company obtained the registration certificate for launching its CMPI in China. May 2008 - The Company obtained FDA510(k) Certificate issued by the FDA for its CMPI angiographic syringes. February 2009 - The Company obtained the registration certificate for launching its disposable pressure transducers in China. August 2009 - The Company obtained FDA510(k) Certificate issued by the FDA for its disposable pressure transducers. July 2011 - The Company obtained FDA510(k) Certificate issued by the FDA for its inflation devices and inflation device compact pack. 2011 - Annual revenue of the Company reached RMB100.0 million. May 2012 - The Company obtained the registration permission for its CMPI high pressure syringes to launch in Canada. September 2012 - The Company obtained a new National High-tech Enterprise Certificate. February 2014 - The Company obtained FDA510(k) Certificate issued by the FDA for its pressure connecting tubes. August 2014 - The Company obtained the registration certificate for launching its dental implant systems in China. . June 2015 - The Company obtained a new National High-tech Enterprise Certificate. July 2015 - The Company completed the construction of its research, development and production facility located in Pingshan New District, Shenzhen. 2017 - Annual revenue of the Company reached RMB300.0 million. February 2018 - Shenzhen Ant Hi-Tech Industrial Co., Ltd. is now a joint stock limited company under the new name Shenzhen Antmed Co., Ltd.

Our Certifications
Showcasing our professional qualifications and recognized industry certifications.